Abstract
Introduction Liquid biopsies leveraging circulating tumor DNA (ctDNA) are rapidly emerging as minimally invasive tools to detect, genotype, and monitor diverse tumors. ctDNA dynamics and early responses are well-characterized in non-Hodgkin Lymphomas, especially during frontline immunochemotherapy. However, ctDNA responses and minimal residual disease (MRD) are less well-defined in classical Hodgkin Lymphomas (cHL), especially in the relapsed/refractory setting and after cellular immunotherapy, where significant unmet needs remain. We hypothesized that ultrasensitive ctDNA-based MRD detection by Phased Variant Enrichment & Detection Sequencing (PhasED-Seq; Kurtz et al, 2021 Nat Biotech) can accurately track response and predict progression after investigational therapy of r/r cHL with autologous CD30.CAR-T cells.
Method This Phase 2 single arm, multicenter study (NCT04268706) investigates safety and efficacy of CD30.CAR-T cells in cHL patients experiencing progression after at least 3 lines of therapy. In the Pilot part of the study, ctDNA was analyzed as an exploratory biomarker using PhasED-Seq at multiple time-points, including at baseline (pre-treatment), Day 42 post-CD30.CAR-T infusion (D42), and upon progressive disease (PD). Exploratory analyses were performed to assess the predictive role of ctDNA and to compare metabolic tumor response by positron emission tomography (PET) scan with ctDNA-based minimal residual disease (MRD). We profiled 55 serial blood specimens (41 plasma and 14 buffy coat samples) from 14 patients with available samples. Cell-free DNA was profiled by PhasED-Seq in the Foresight Diagnostics CLIA laboratory (Aurora, CO). Baseline plasma and D42 buffy coat specimens were used to genotype each patient's tumor to identify Phased Variants (PVs), and these were used to monitor MRD in subsequent blood specimens. Patients/samples were reported as MRD positive when ctDNA levels exceeded an analytical detection threshold (~1 part per million cfDNA molecules), corresponding to 98% clinical specificity.
Result As of 22 July 2022, 17 patients have been screened and 15 patients enrolled in the Pilot segment (median age: 35 years [21-57], 66.7% male). Overall response rate (ORR) after CD30.CAR-T single infusion was 73.3% (n=11), with complete response (CR) rate of 60% (n=9). ORR in 5 patients after the 2nd infusion was 100% (n=5), with CR of 60% (n= 3).
ctDNA was successfully genotyped directly from pre-treatment plasma in 12 out of 14 patients where samples were available. Both patients where PV genotyping failed had received bridging cytoreductive therapy before baseline plasma collection. Out of 12 profiled patients, 7 patients achieved CR with median baseline ctDNA concentration of 44.9 hGE/mL. 3 patients had PD with median baseline ctDNA level of 94.0 hGE/mL. One patient with PR and one patient with SD had baseline ctDNA levels of 38.9 hGE/mL and 231.1 hGE/mL, respectively. These results suggest that pre-treatment ctDNA levels could have predictive value on patient response to CAR-T therapy.
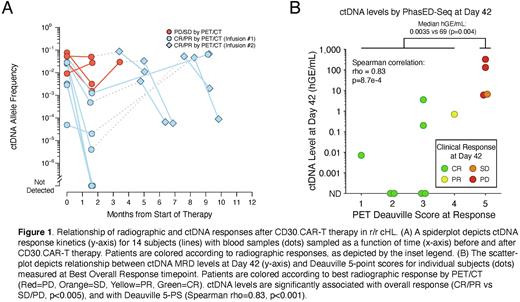
ctDNA responses largely mirrored radiographic responses. Specifically, among 7 patients achieving radiographic CR, 4 also achieved undetectable ctDNA, and the other 3 had ctDNA responses with low MRD levels (median: 0.2 hGE/mL) at D42. One patient with PR also had low ctDNA concentration at D42 (0.7 hGE/mL). In comparison, 3 patients experiencing PD had relatively higher ctDNA levels (median: 131.4 hGE/mL). Furthermore, ctDNA levels at D42 were significantly correlated with PET Deauville score (p=8.7e-4), and patients with a response had significantly lower ctDNA than those without a response (p=0.004) (Fig B). These results suggest that ctDNA-MRD levels are strongly correlated with metabolic responses determined by PET imaging. Similar results were observed after 2nd infusions of the CAR-T. 3 out of 3 patients achieving CR had low ctDNA concentrations at Day 42 post-2nd infusion (median: 0.2 hGE/mL). Among 2 patients with PR, 1 patient with available sample had 31.9 hGE/mL ctDNA.
Conclusion Minimally invasive ctDNA analysis is a viable method to monitor responses, rapidly risk stratify and predict outcomes of patients with r/r cHL treated with CD30.CAR-T therapy. A single infusion of CD30.CAR-T therapy rapidly induces MRD negativity in a significant subset of patients at Day 42. ctDNA as exploratory biomarker will be further evaluated in Pivotal part of the study.
Disclosures
Kurtz:Genentech: Consultancy; Roche: Consultancy; Adaptive Biotechnologies: Consultancy; Foresight Diagnostics: Consultancy, Current equity holder in private company, Patents & Royalties. Ahmed:Xencor: Research Funding; Tessa Therapeutics: Consultancy, Research Funding; Seagen: Research Funding; Chimagen: Consultancy, Research Funding; Servier: Membership on an entity's Board of Directors or advisory committees; Myeloid Therapeutics: Consultancy; Merck: Research Funding. Tong:Tessa Therapeutics: Current Employment; Neurona Therapeutics: Current holder of stock options in a privately-held company. Mei:CTI: Honoraria; Novartis: Consultancy; Incyte: Research Funding; Beigene: Research Funding; Celgene: Research Funding; EUSA: Honoraria; Morphosys: Research Funding, Speakers Bureau. Ding:Tessa Therapeutics: Current Employment. Flinn:Pharmacyclics: Consultancy, Research Funding; Fate Therapeutics: Research Funding; CALIBR: Research Funding; Kite Pharma: Consultancy, Research Funding; Nurix Therapeutics: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; MorphoSys: Consultancy, Research Funding; CALGB: Research Funding; Tessa Therapeutics: Research Funding; Servier Pharmaceuticals: Consultancy; AstraZeneca: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; Iksuda Therapeutics: Consultancy; ArQule: Research Funding; Pfizer: Research Funding; Century Therapeutics: Consultancy; Genentech: Consultancy, Research Funding; Genmab: Consultancy; Unum Therapeutics: Research Funding; Vincerx Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Acerta Pharma: Research Funding; Agios: Research Funding; Curis: Research Funding; Portola Pharmaceuticals: Research Funding; Rhizen Pharmaceuticals: Research Funding; CTI Biopharma: Research Funding; Celgene: Research Funding; Incyte: Research Funding; Epizyme: Research Funding; TG Therapeutics: Consultancy, Research Funding; City of Hope National Medical Center: Research Funding; Abbvie: Consultancy, Research Funding; Verastem: Consultancy, Research Funding; InnoCare Pharma: Consultancy, Research Funding; Forma Therapeutics: Research Funding; Merck: Research Funding; Loxo@Lilly: Research Funding; Infinity Pharmaceuticals: Research Funding; Hutchison MediPharma: Consultancy; Forty Seven: Research Funding; IGM Biosciences: Research Funding; Constellation Pharmaceuticals: Research Funding; Trillium Therapeutics: Research Funding; Myeloid Therapeutics: Research Funding; Triphase Research & Development Corp: Research Funding; 2seventy bio: Research Funding; Seattle Genetics: Research Funding; Takeda: Consultancy; TCR2 Therapeutics: Research Funding; Secura Bio: Consultancy; Millenium Pharmaceuticals: Research Funding; Novartis: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Bristol Myers Squibb: Research Funding; Biopath: Research Funding; Xencor: Consultancy; Gilead Sciences: Research Funding. Riedell:Sana Biotechnology: Consultancy; Nurix Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BeiGene: Consultancy; Intellia Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Xencor: Research Funding; Calibr: Research Funding; Tessa Therapeutics: Research Funding; MorphoSys: Research Funding; Fate Therapeutics: Research Funding; Nektar Therapeutics: Membership on an entity's Board of Directors or advisory committees. Hogan:Foresight Diagnostics: Current Employment; Freenome: Other: Equity holder. Schultz:Foresight Diagnostics: Current Employment. Chabon:Foresight Diagnostics: Current Employment, Current holder of stock options in a privately-held company. Heslop:Kiadis: Divested equity in a private or publicly-traded company in the past 24 months; Marker Therapeutics: Current equity holder in publicly-traded company; Allovir: Current equity holder in publicly-traded company; Kuur Therapeutics: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Gilead Biosciences: Membership on an entity's Board of Directors or advisory committees; Immunai: Membership on an entity's Board of Directors or advisory committees; Ankarys: Membership on an entity's Board of Directors or advisory committees; Millenium: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Fresh Wind Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Tessa Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Myo:Tessa Therapeutics: Current Employment. Alizadeh:Adaptive Biotechnologies: Consultancy; Gilead: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months, Patents & Royalties; Syncopation: Current equity holder in private company, Patents & Royalties; Roche: Consultancy; BMS: Consultancy, Research Funding; Genentech: Consultancy; Karyopharm: Consultancy; Cibermed Inc: Consultancy, Current equity holder in private company, Patents & Royalties; Foresight Diagnostics: Consultancy, Current equity holder in private company, Patents & Royalties. Horak:Tessa Therapeutics: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal